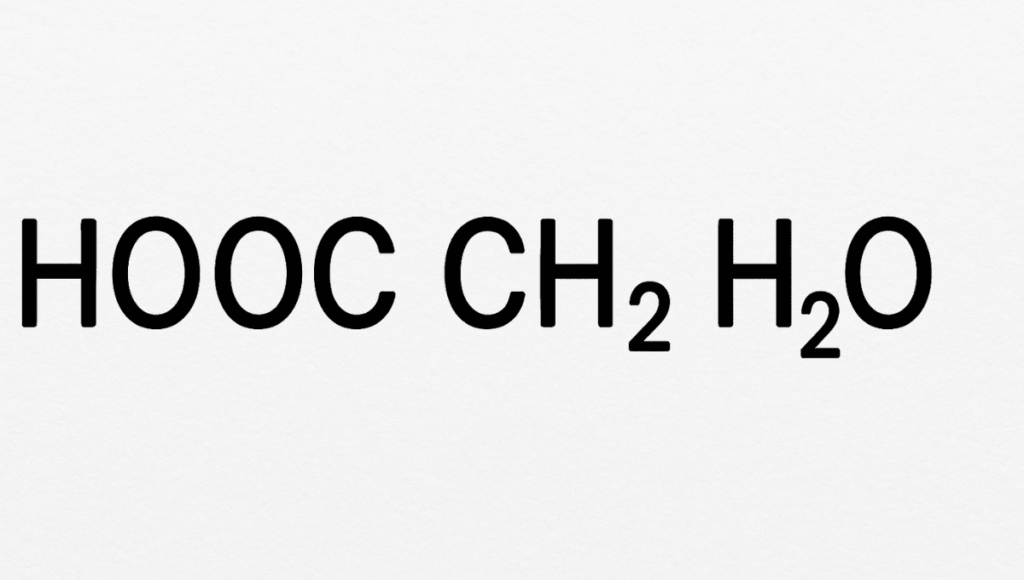- Understanding HCOOCH CH2 H2O
- To understand it more, let’s look at what each part represents
- The part of HCOOCH CH2 H2O in Organic Chemistry
- Real- World operations of HCOOCH CH2 H2O
- Breaking Down the response
- Why HCOOCH CH2 H2O Matters for scholars?
- Industrial Importance of HCOOCH CH2 H2O
- Environmental and Safety Aspects
- The Future of Research Involving HCOOCH CH2 H2O
If you’ve been curious about HCOOCH CH2 H2O, you aren’t the only individual. This chemical formula might look a little confusing at first regard, but it has a fascinating place in organic chemistry and is connected to important responses used in labs and diligence. Whether you’re a pupil trying to make sense of it, a chemistry sucker, or someone exploring chemical processes for practical use, this companion will help you understand what HCOOCH CH2 H2O means, how it’s formed, and why it matters.
Let’s take a deep dive into its composition, responses, and operations so that you get a clear and friendly explanation — without feeling overwhelmed by specialized slang.
Understanding HCOOCH CH2 H2O
First, let’s break down HCOOCH CH2 H2O step by step. In simple terms, this is a way of writing a patch that can be understood as a combination of different groups, formate( HCOO-), methylene( CH2), and water( H2O). When written like this, it generally refers to a specific type of ester or intermediate emulsion set up in certain chemical responses.
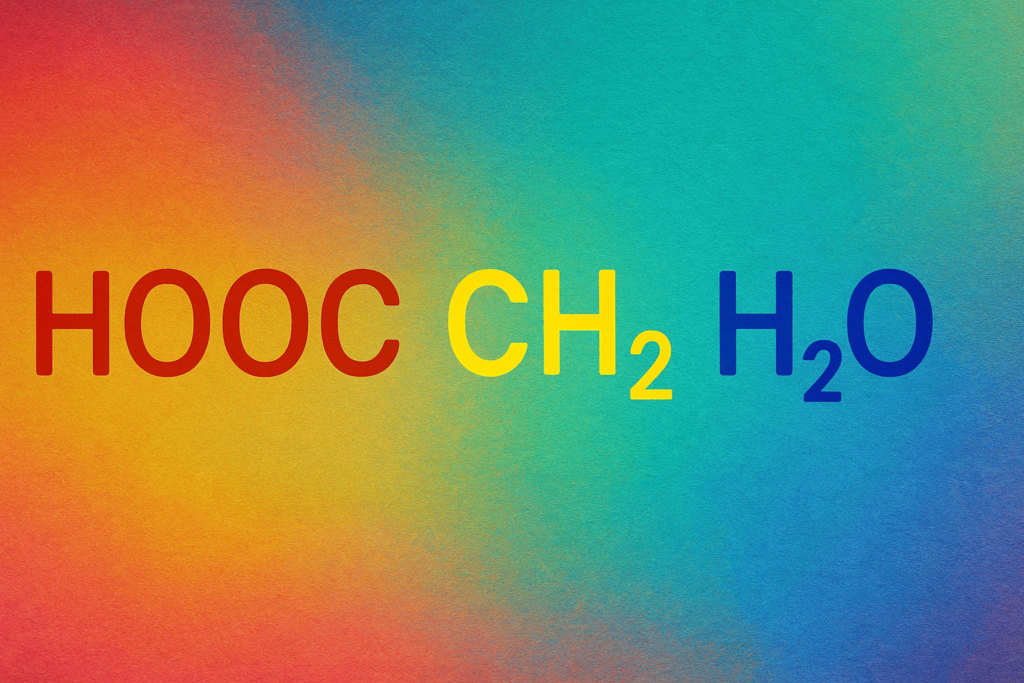
In organic chemistry, composites like this are frequently involved in hydrolysis responses where water( H2O) is used to break a patch into lower factors. That’s why the presence of H2O in the formula is significant. It hints that this might be a hydrate or a response product involving water.
To understand it more, let’s look at what each part represents
- HCOOCH – This is related to a formate ester group, where formic acid reacts with an alcohol group.
- CH2 – A methylene group that serves as a linking carbon.
- H2O – Water patch, either free or part of a response admixture.
Chemistry scholars frequently encounter this type of patch when they study esterification, hydrolysis, or indeed biochemical pathways.
The part of HCOOCH CH2 H2O in Organic Chemistry
Now that we’ve an introductory idea of its composition, let’s talk about why HCOOCH CH2 H2O matters. Organic chemistry is full of motes that might look intimidating but actually follow logical rules. This particular emulsion or response is a great illustration of how druggists make bigger motes from lower bones and how they can break them down again when demanded.
In the lab, composites like this might be used to produce formates, which are important in synthetic chemistry. They can also appear as interceders when producing certain flavors, spices, and medicinals.
One important concept to keep in mind is ester hydrolysis.However, adding water( H2O) can break the ester bond and release formic acid and alcohol derivations, If you start with an emulsion like HCOOCH CH2. This is a common response tutored in organic chemistry classes and is also seen in nature, our own bodies use analogous responses to metabolize motes.
Real- World operations of HCOOCH CH2 H2O
You might be wondering, “ Why should I watch a chemical formula like HCOOCH CH2 H2O if I’m not a druggist? ” The answer lies in how this emulsion or response relates to everyday life.
Formates and related esters are used in.
- Pharmaceutical assiduity – for synthesizing medicine motes.
- Scent and flavor product – to produce fruity or flowery smells.
- Chemical conflation – as interceders in making other useful composites.
- Laboratory education – as classic exemplifications of esterification and hydrolysis.
When you see a chemical name like this in a text or lab primer, it generally represents a step in a bigger picture — a process where simple motes are converted into a more precious commodity.
Breaking Down the response
To make it clearer, let’s imagine you have HCOOCH CH2 as an ester. When you add H2O, a hydrolysis response occurs. This means the bond between the formate group and the CH2 group breaks, producing two lower motes.
This is an acid- catalyzed hydrolysis if you add acid to speed up the process, or a base- catalyzed hydrolysis( also called saponification) if you use a base like NaOH. Both are common in organic chemistry.
Then’s a simplified explanation.
- Start with HCOOCH CH2( the ester).
- Add H2O( water).
- Break the bond.
- Get formic acid( HCOOH) and an alcohol( related to CH2 group).
This response is important not only in labs but also in natural metabolic pathways where esters need to be broken down for energy.
Why HCOOCH CH2 H2O Matters for scholars?
Still, learning about HCOOCH CH2 H2O is an occasion to master a crucial principle: understanding how motes interact with water, if you’re a chemistry student. Hydrolysis is one of the most abecedarian responses you’ll ever study.
Knowing how to write, interpret, and prognosticate the outgrowth of these responses is essential for examinations and for any practical work you might do later. It’s also a stepping stone toward understanding biochemistry, where analogous responses are all the time inside living organisms.
Industrial Importance of HCOOCH CH2 H2O
Beyond the classroom, chemical companies use motes like HCOOCH CH2 H2O in controlled responses to produce precious accoutrements . For instance, formate esters are used as detergents, preservatives, and interceders for plastics and resins.
Since water is involved, druggists must precisely control response conditions, temperature, pressure, and pH — to ensure they get the asked product rather than unwanted derivations.
This is why chemical masterminds spend time optimizing responses that involve hydrolysis or esterification. Indeed a small change in water content can affect the yield of the response significantly.
Environmental and Safety Aspects
Whenever you deal with chemical composites, safety is important. Okay, so HCOOCH2H2O isn’t too scary in tiny lab doses, but what it turns into, like formic acid, can sting. When messing with stuff like this, gloves, goggles, and good air flow are a must.
In assiduity, waste from these responses must be treated to avoid contaminating water sources. Understanding the chemistry helps to help accidents and cover the terrain.
The Future of Research Involving HCOOCH CH2 H2O
Chemistry is an evolving field, and motes like this continue to play a part in exploration. As green chemistry becomes further popular, scientists are changing new ways to make and break esters usingeco-friendly catalysts and renewable coffers.
So, using HCOOCH CH2 H2O in reactions could be cleaner, faster, and better for the environment—that’s great for business and the planet too.
Conclusion
Okay, so HCOOCH CH2 H2O might seem like a weird code at the start, but once you get what it means, it gives you a peek into organic chemistry. It shows you about esters, hydrolysis, and how water plays a role in stuff reacting.
Whether you’re a pupil, an experimenter, or just someone curious about wisdom, understanding this patch gives you sapience into a process that affects everything from medicine manufacturing to the flavors in your food. The next time you come across HCOOCH CH2 H2O, you’ll know it’s further than just a formula, it’s a story of chemistry in action.